A UNIQUE MICROCOSM METHOD TO ASSESS THE
MICROBIAL COMMUNITY AT ANAEROBIC BIOREMEDIATION SITES
Samuel Fogel, Ph.D.(SFogel@bciLabs.com)
Margaret Findlay, Ph.D., and Donna Smoler,
(Bioremediation Consulting, Inc., Watertown, MA, USA)
Bradley F. Droy, Ph.D., Frank Manale, and Peikang Jin, Ph.D., P.E.,
(Toxicological & Environmental Associates, Inc., Baton Rouge, LA, USA)
Catherine Creber, (The Dow Chemical Company, Sarnia, Ontario Canada)
Gary Klecka, Ph.D., (The Dow Chemical Company, Midland, MI, USA)
ABSTRACT: A microcosm method is described for detecting the presence of anaerobic bacteria which are important for the degradation of chlorinated compounds. Sulfate-reducing bacteria, PCE-degraders, DCE-degraders (ethenogens), carbon tetrachloride (CT)-degraders, and methanogens were assessed in microcosms constructed from site groundwater and soil from 17 locations at the Dow-Pittsburg bioremediation site. The test for each location involved five microcosms which were initially sparged with oxygen-free gas to remove native mixtures of chlorinated compounds. Four microcosms were provided with organic acids as electron donor, and allowed to reduce the native sulfate (400 to 900 mg/L), while the fifth was killed as a control. After sulfate reduction, the three test compounds were added to separate microcosms, and the fourth microcosm, without contaminant, was used to detect methanogens. Results of microcosm tests for the 17 well locations which contained sulfate-reducing bacteria showed CT-degraders in 14 locations, PCE-degraders in 13, methanogens in 6, and DCE-degraders in 8 locations. These results were used to design an intra-site bioaugmentation system. Similar microcosm tests using well samples (without soil) are being used to track the movement of bacteria to monitoring wells during bioremediation.
INTRODUCTION
The Dow Chemical Company is conducting in-situ anaerobic bioremediation for 200 acres of their operating chemical plant in Pittsburg, California. Concomitant biostimulation and bioaugmentation are being used to optimize biodegradation of PCE and its daughter products as well as carbon tetrachloride (CT) and its daughter products. The treatment system comprises 39 wells that circulate groundwater and amendments creating a bioactive zone that extends 3,800 feet across the plume. Previously, a chemical survey of terminal electron acceptors in the proposed remedial area identified iron reduction, sulfate reduction, and methanogenesis as significant processes at this site. The presence of daughter products indicated that dechlorination had occurred, but could not indicate whether dechlorination was on-going, or whether it was occurring up-gradient or at the sampling location.
We describe anaerobic microcosm testing which confirms and expands these observations, and provides detailed information on the microbial community involved in reductive dechlorination at the site. The test results show that the site microbial community includes active sulfate-reducing bacteria, PCE-degraders, DCE-degraders, CT-degraders and methanogens, and indicates which of these microbial types are most active in specific locations in the bio-active zone. An important feature of the test is sparging of the site sample to remove the mixture of native contaminants, and adding back only one chlorinated compound per microcosm. Also, because this test is designed to detect microbial types regardless of the presence of electron donor in the site sample, organic substrate is added to the microcosms. We describe this unique microcosm method, as well as some of the findings concerning the distribution of important microbial types at the Dow-Pittsburg site.
MATERIALS AND
METHODS
Bottles for ground water were prepared by filling with
oxygen-free gas (Argon), and were provided with FeS reducing agent (to give 0.4
mM) to react with traces of oxygen that might enter during sampling. Soil cores were obtained in 6”
brass cylinders, capped and taped immediately, and shipped to the
Bioremediation Consulting laboratory, where cores were transferred to anaerobic
chambers maintained in anoxic atmosphere (4 % H2 in Argon). For each Microcosm Set, comprising
several microcosms from the same well location, 160 ml serum bottles were
flushed with Argon, stoppered, and placed in a glove box, together with the
corresponding soil core, and the glove box was flushed with 4 % H2
in Argon in the presence of catalyst to provide an oxygen-free atmosphere.
Soil, 30 grams, were placed in each bottle, and the bottles stoppered. Bottles were then removed from the
glove box and provided with 90 ml of ground water using anoxic transfer
procedures involving continuous flushing of the groundwater sample and of the
microcosm bottle with argon. Microcosms were then sealed with Teflon-lined
butyl rubber stoppers, crimped, and overpressurized.
Amendment solutions were prepared using anoxic procedures, and additions were made using anaerobic technique. One microcosm in each set was killed by adding sufficient 6N HCl to reduce the pH below 3, to create a control. Each live microcosm was amended to give 180 mg/L each of formate and lactate, 30 mg/L ammonium-nitrogen, 60 mg/L phosphate, and given 5 mg yeast extract and 5 micrograms vitamin B12. Microcosms were sparged with ultra-pure Argon to remove chlorinated solvents, then briefly with 80% N2 /20% CO2 to replace Argon. After sulfate-reducing bacteria had reduced the sulfate concentration from the native 500 to 900 mg/L to less than 100 mg/L, one microcosm in each set was spiked to give 5 ppm of either PCE, CT or cDCE, and the Killed Control was spiked with all three test compounds.
Microcosms were maintained in darkness, inverted, at 22 ± 1 oC , and shaken briefly 3 times per week. Additional lactate and formate were added during the test, as necessary, to support complete sulfate reduction and maintain dissolved H2. On day 60, additional B12 was added. Microcosms were monitored over 60-90 day periods. PCE and its dechlorination products TCE, cDCE, VC and ethene, CT and its dechlorination products CF and DCM (dichloromethane), as well as methane, were analyzed by removing 100 mL samples from the microcosm headspace and injecting into a HP5890 gas chromatograph with flame ionization detector. Sulfate and organic acids were determined by removing 100 mL aqueous samples and analyzing using capillary ion electrophoresis. Dissolved molecular H2 was determined by removing 100 mL headspace samples, diluting with argon, and analyzing using a Trace Analytical reduction gas analyzer.
RESULTS
Table 1 presents data on dechlorinators and methanogens for 17 locations which contained sulfate reducers. Rates of sulfate reduction ranged from 30 to 100 mg/L/day. Of the 17 locations showing sulfate reduction, 13 had PCE-degraders, and 8 contained ethenogens. CT degraders were found at 14 locations. For four locations, CT was converted to chloroform (CF) on a molar basis. For ten locations, only 20 to 50 % of CT could be accounted for as CF, indicating that the remainder was converted to CO2. Methanogens were found at 7 locations.
TABLE 1 Microorganisms active in anaerobic soil/groundwater
microcosms
from 17 locations at the Dow Pittsburg site
|
Well Location |
Sulfate Reducers |
CT Degraders |
PCE Degraders |
DCE De- chlorinators |
Methan- ogens |
|
PZ 000B |
x |
x |
x |
x |
x |
|
PZ 000C |
x |
x |
x |
|
x |
|
PZ 002B |
x |
x |
x |
|
|
|
PZ 002C |
x |
x |
x |
|
|
|
PZ 004B |
x |
x |
x |
x |
x |
|
PZ 004C |
x |
x |
x |
x |
x |
|
PZ 500B |
x |
x |
x |
|
|
|
PZ 500C |
x |
x |
x |
|
|
|
PZ 503B |
x |
|
|
|
|
|
PZ 503C |
x |
x |
|
|
|
|
PZ 600B |
x |
|
x |
x |
|
|
PZ 609C |
x |
x |
x |
x |
|
|
BW 612 |
x |
x |
x |
x |
x |
|
PZ 619B |
x |
x |
x |
x |
x |
|
PZ 621B |
x |
|
|
|
x |
|
PZ 621C |
x |
x |
x |
x |
|
|
PZ 623C |
x |
x |
|
|
|
One location, BW-612 showed the presence of six physiologic groups of bacteria: sulfate-reducers, PCE- and DCE-degraders, CT-and CF-degraders, and methanogens. The results from microcosm studies from BW-612 are shown in Figure 1. The top graph shows that 525 mg/L of sulfate was reduced in less than 13 days. Methane first appeared only after complete sulfate reduction.
The middle graph shows the result of spiking 5 mg/L of CT into a microcosm after sulfate reduction. The results show stoichiometric conversion of CT to CF and DCM. (Separate experiments with material from this location, not presented, show dechlorination of CF to DCM, and further to non-chorinated end-products.)
The bottom graph shows the result of spiking 5 mg/L of PCE into a microcosm after sulfate reduction. PCE was converted completely to ethene in 60 days indicating the presence of both PCE-degraders and ethenogens.
In the killed control microcosm, the concentrations of the added test compounds, PCE, cDCE, and CT, remained constant during the entire test period (data not shown).
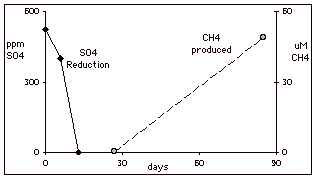

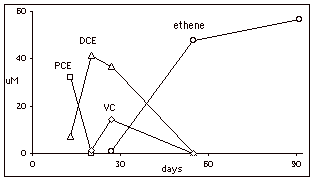
FIGURE 1. Microcosm results from Well BW-612
amended with lactate and formate as electron donors. Top: Sulfate reduction in all microcosms, and methane
formation in the un-spiked microcosm.
Middle: Microcosm
spiked with 5 ppm CT, day 13. Bottom:
Microcosm spiked with 5 ppm PCE, day 13.
DISCUSSION AND CONCLUSIONS
The Dow-Pittsburg site contains, in the subsurface, two major groups of chlorinated compounds, the chloroethenes and chloromethanes. The results of this investigation show that the soils and groundwater from this site contain a wide variety of microorganisms capable of biodegrading all major contaminants. In addition, the microbial community has been found to include methanogens and sulfate-reducing bacteria. The presence of methanogens is important since these organisms can serve as a source of vitamins and co-factors for the dechlorinators. The sulfate reducers are particularly important at Pittsburg site because concentrations of sulfate are as high as 900 mg/L.
The microcosm results show that the critical microbial types are distributed across the bioactive zone. Microorganisms from one location can readily be used to bioaugment adjacent locations by transfer of groundwater within the circulation system.